For about two weeks that Abiodun (surname withheld), a 22-year-old sickle cell carrier, spent on the hospital bed when he had another round of the ‘usual’ crisis, his father and a few family members around him struggled to keep hope alive, mumbling to themselves even as they watched him shuttle between life and death in excruciating pain.
The dawn and dusk of each day met him in bed. And on the night of his 13th day in the hospital, somewhere in Lagos, after all efforts to save his life failed, his father’s disapproving frown after the doctor whispered the dreaded news to him, relayed the message to all. He was gone. And across the room, the incident reduced almost everyone to tears. He was the second child the parents would lose in three years.
It was yet another death from the disease, which is said to have its root in Africa and the most common blood disorder in many countries across continents, affecting millions of people and sending some to early grave. The World Health Organisation said in a report that approximately five per cent of the world’s population carries trait genes for haemoglobin disorders.
Ogbonna et al in an article published in the Pan-African Medical Journal in February 2022 said about 50 million people live with sickle cell disorder globally and that Nigeria remained the epicentre, with between four and six million people. The researchers added that every year, not less than 300,000 children are born with the disease and Nigeria accounts for 100,000 to 150,000 newborns.
“Sickle cell disease poses significant challenges to global population health. It contributes significantly to the morbidity and mortality of paediatric and adult population,” they noted.
Sadly too, they found that about 50 to 90 per cent of children born with the disease in low- and low-middle-income countries of sub-Saharan Africa die before their fifth birthday, and that it accounts for 20 per cent of neonatal mortality and five per cent of mortality of under-five children in Africa.
In addition to the enormous pain, they experience stunted growth, delayed puberty, swollen hands and feet, and frequent infections, among others.
Meanwhile, despite its prevalence and its contribution to deaths globally, the only popular treatment for sickle cell so far is stem cell transplant, which, according to doctors, is expensive and has its associated risks and challenges.
Synthego, a genome engineering company, affirmed that treatment options had been limited, noting that the condition is usually managed, adding, “The only cure currently available for sickle cell disease is the transplantation of bone marrow from a healthy donor. This approach presents significant challenges, including identifying a suitable donor, immune rejection of the transplant, and graft-versus-host disease. It is also typically restricted to children and young people because the associated risks increase with the age of the patient.”
Gene editing as the way out
Meanwhile, help seems to be on the way, with the advent of CRISPR gene therapy. In fact, the duo of Jennifer Doudna and Emmanuelle Charpentier won the Nobel Prize in Chemistry in 2020 for the CRISPR/Cas9, which has been described as one of gene technology’s sharpest tools.
In its article titled, ‘Genetic scissors: a tool for rewriting the code of life’, the organisers of the prize said, “Researchers are already performing clinical trials to investigate whether they can use CRISPR/Cas9 to treat blood diseases such as sickle cell anaemia and beta thalassemia, as well as inherited eye diseases.”
A research instructor at the Matthew Porteus’ laboratory at Stanford University, United States, Dr Daniel Dever, who has used CRISPR to introduce a DNA break to the ß-globin gene, noted that one of the main approaches to CRISPR sickle cell gene therapy is to repair the mutation in the adult haemoglobin gene responsible for sickle cell disease, causing the healthy, normal form of adult haemoglobin (hemoglobin S) to be produced.
Dever, who has completed his preclinical work, said the site of the break could be used to introduce a correction to the gene via homology-directed repair, a process known as knock-in.
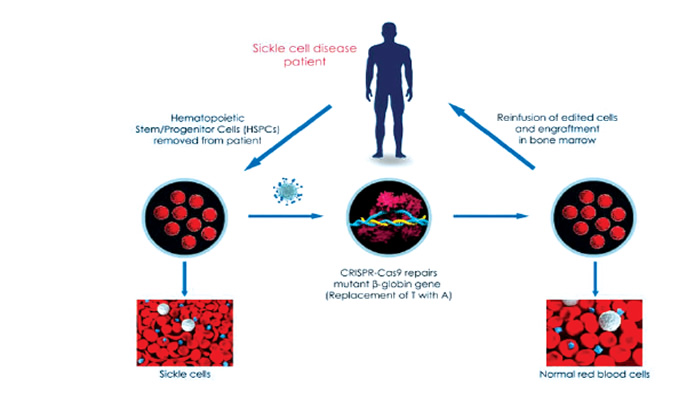
Also, according to Synthego, treating sickle cell anemia with CRISPR involves an ex vivo procedure, known as gene-edited cell therapy, where hematopoietic stem cells are extracted from the patient, corrected and then replaced.
Interestingly, there are six CRISPR sickle cell gene therapy trials already, out of which the CTX001, which seeks to restore fetal hemoglobin by knocking out BCL11A transcription factor in autologous CD34+ HSPCs via CRISPR-Cas9, was adjudged complete and successful. However, the remaining five, including OTQ923 and HIX763, ET-01, EDIT-301, CRISPR_SCD001 and then GPH101, which seeks to correct the mutation in HBB to restore normal hemoglobin expression, are at different phases.
Synthego noted that Victoria Gray, who had fought the disease for 34 years, was the first CRISPR sickle cell patient, adding that after the one-time treatment, her blood showed a significant proportion of fetal hemoglobin levels and she had lived without blood transfusions and pain attacks, without any major side effects.
The Medical Director, pediatric hematology/oncology at HCA Healthcare’s Sarah Cannon Research Institute Centre at Tennessee, US, Dr Haydar Frangoul, treating Gray, said, “She is functioning as somebody who does not have sickle cell disease. I believe this is absolutely, totally transformative therapy.”
The report also noted that at the American Society of Hematology annual meeting in 2020, data presented showed that a total of 10 patients – three with sickle cell and seven with beta-thalassemia, showed tremendous progress. In June, 2021, the researchers presented more long-term results of 22 patients at the European Hematology Association annual meeting, demonstrating consistent and sustained responses from the patients following treatment. “With such promising data, this treatment approach is looking more promising to become mainstream,” it added.
A consultant endocrinologist, Dr Michael Olamoyegun, who sounded upbeat about the prospect of using CRISPR-Cas9 to treat sickle cell, told The PUNCH in an interview that the prospect of the technology earned the researchers the Nobel Peace Prize.
Narrating how the technology would work, Olamoyegun, an Associate Professor of medicine, said since amino acid was the problem, it could be removed and replaced.
He explained, “In sickle cell, the basic problem is the haemoglobin. There is the substitution of one amino acid by another; the substitution of glutamic acid by valine. That is the basic problem. Due to that, the red cell that should normally last for 120 days dies about 21 to 30 days, which is why they normally have low blood levels.
“So, since the problem is the amino acid, the question is, can’t we change that amino acid? Can’t we remove that amino acid and insert a healthy one as a replacement? That is what the gene editing seeks to do. It would look at where the abnormal cell is, cut it off and replace it with a new one, and with that, sickle cell would be curable in the future.”
Also, a professor of Haematology and former president, Nigerian Society of Haematology and Blood Transfusion, Aisha Kuliya-Gwarzo, said she was positive that the CRISPR gene editing technology might be able to solve the genetic disorder.
She told The PUNCH, “CRISPR has the potential to correct this genetic mutation by editing the DNA sequence of the patient and replacing the mutated gene with a healthy gene that would be inserted. Potentially, it can offer a solution to sickle cell disease.
“Since it is a genetic disorder, if you find a way to delete the gene, it means people would no longer carry the gene, and it means it cannot be transferred. It has the potential to give us a sickle cell-free society. However, it has not been approved for use, but we will see more possibilities when the time comes.”
She said even though the cost might be high if eventually approved and deployed, insurance would help, since the disease is of global concern.
The don advised that the best way out is for people who want to get married to make informed decisions with regard to their genotype, adding that early detection was better.
Also, a professor in haematology, Faculty of Basic Clinical Sciences, Nnamdi Azikiwe University, Emmanuel Okocha, described stem cell transplant as a solution that could potentially cure sickle cell disease but that it is expensive and has its side effects and complications.
He added, “The gene editing is an emerging solution, and the technology is that it can enter the cell and change the genetic problem that is causing the disease such that the blood cells would come out normal. Instead of SS, the individual becomes AA or AS, depending on how you edit it. So, what we know is that it’s an emerging technology.
“However, I will also canvass for the use of indigenous solutions to the disease. There are potential herbal remedies that could be looked into. We need self reliance and we must find a way to collaborate to harness what we have.”





